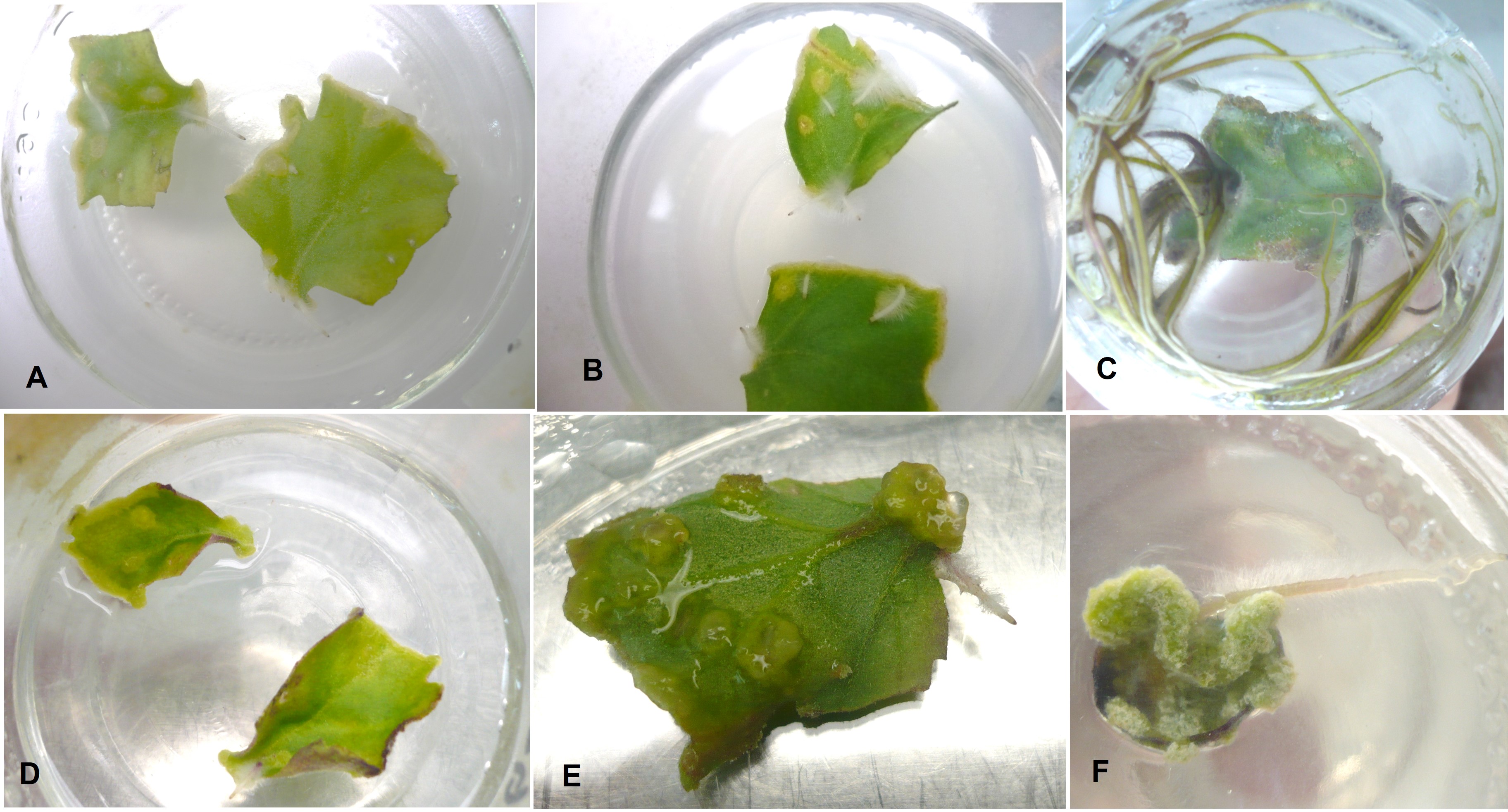
MORPHOGENIC RESPONSE FROM LEAF EXPLANT OF Dendranthema grandiflora VAR MICROMARGARA
Abstract
Keywords
Full Text:
PDFReferences
Ali, H.M., Khan, T., Khan, M.A. and Ullah, N., 2022. The multipotent thidiazuron: A mechanistic overview of its roles in callogenesis and other plant cultures in vitro. Biotechnology and Applied Biochemistry, 69(6), 2624-2640. https://doi.org/10.1002/bab.2311.
Babich, O., Sukhikh, S., Pungin, A., Ivanova, S., Asyakina, L. and Prosekov, A., 2020. Modern Trends in the In Vitro Production and Use of Callus, Suspension Cells and Root Cultures of Medicinal Plants. Molecules, 25, pp- 5805. https://doi.org/10.3390/molecules25245805.
Babiker, Y.F., Eltorky, Elmokadem, El-Naggar and Meheissen, M.A.M., 2021. Establishment of Callus Cultures of Chrysanthemum (Dendranthema × grandiflorum) var. ‘Zembla yellow’. Journal of Agriculture Science, 66 (5), pp. 123-132.
Bety, Y.A. and Pangestuti, R., 2021. IOP Conference Series: Earth and Environmental Science Resistance varieties and pattern of disease progress of rust (Pucciana horiana p. henn) in Chrysanthemum. IOP conference series: Earth and Enviromental Science, 883, pp. 012023. https://doi.org/10.1088/1755-1315/883/1/012023.
Chowdhury, J., Hoque, M. and Sarker, R., 2021. Development of an Efficient In vitro Regeneration Protocol for Chrysanthemum (Chrysanthemum morifolium Ramat). Plant Tissue Culture and Biotechnology, 31(2), pp. 161–171. https://doi.org/10.3329/ptcb.v31i2.57344.
Delgado-Aceves, L., González-Arnao, M.T., Santacruz-Ruvalcaba, F., Folgado, R. and Portillo, L., 2021. Indirect somatic embryogenesis and cryopreservation of agave tequilana weber cultivar ‘chato’. Plants, 10(2), pp. 249. https://doi.org/10.3390/plants10020249.
Delporte, F., Pretova, A., du Jardin, P. and Watillon, B., 2014. Morpho-histology and genotype dependence of in vitro morphogenesis in mature embryo cultures of wheat. Protoplasma, 251, pp. 1455–1470. https://doi.org/10.1007/s00709-014-0647-7.
Din, A., Qadri, Z.A., Wani, M.A., Iqbal, S., Malik, S.A., Bhat, Z.A. and Banday, N., 2022. Developing an efficient in vitro callusing and regeneration protocol in Dendranthema × grandiflorum Kitam. Journal of Crop Science and Biotechnology, 25, 393-405. https://doi.org/10.1007/s12892-022-00140-w.
Fritsche, Y., Stefenon, V.M., Lovato, P. e. and Guerra, M.P., 2022. TDZ induces a dual morphogenetic pathway on leaf explants of the beach huckleberry – Gaylussacia brasiliensis. South African Journal of Botany, 145, pp. 468-472. https://doi.org/10.1016/j.sajb.2022.03.007.
García-Hernández, E., Loera-Quezada, M.M., Morán-Velázquez, D.C., López, M.G., Chable-Vega, M.A., Santillán-Fernández, A., Zavaleta-Mancera, H.A., Tang, J.Z., Azadi, P., Ibarra-Laclette, E., Ibarra-Laclette, E. and Alatorre-Cobos, F., 2022. Indirect organogenesis for high frequency shoot regeneration of two cultivars of Sansevieria trifasciata Prain differing in fiber production. Scientific Reports, 12(1), pp. 8507. https://doi.org/10.1038/s41598-022-12640-4.
Ghosh, S., B.N. Naika, M., Nishani, S., Shiragur, M. and Bhat, A., 2018. Studies on Somatic Embryogenesis in Chrysanthemum cv. Marigold Using Root and Leaf as Explants. International Journal of Current Microbiology and Applied Sciences, 7(08), pp..3965–3971. https://doi.org/10.20546/ijcmas.2018.708.409.
Haradzi, N.A., Khor, S.P., Subramaniam, S. and Chew, B.L., 2021. Regeneration and micropropagation of Meyer lemon (Citrus x meyeri) supported by polymorphism analysis via molecular markers. Scientia Horticulturae, 286, pp. 1-11. https://doi.org/10.1016/j.scienta.2021.110225.
Hodson De Jaramillo, E., Forero, A., Cancino, G., Moreno, A.M., Monsalve, L.E. and Acero, W., 2008. In vitro regeneration of three Chrysantemum (Dendrathema grandiflora) varieties via organogenesis and somatic embryogenesis. [online] Universitas Scientiarum, 13, pp.118-127. Available at: http://www.javeriana.edu.co/universitas_scientiarum
Ikeuchi, M., Sugimoto, K. and Iwase, A., 2013. Plant callus: Mechanisms of induction and repression. Plant Cell, https://doi.org/10.1105/tpc.113.116053.
Jin, L., Yang, Y., Gao, W., Gong, M., Wang, J., O. Anderson, N. and He, M., 2017. Establishment of Callus Induction and Cell Suspension Cultures of Dendrathema Indicum var. Aromaticum a Scented Chrysanthemum. Journal of Plant Studies, 6(2), p. 38. https://doi.org/10.5539/jps.v6n2p38.
Kazeroonian, R., Mousavi, A., Kalate Jari, S. and Tohidfar, M., 2018. Factors Influencing in Vitro Organogenesis of Chrysanthemum morifolium cv. ‘Resomee Splendid’. Iranian Journal of Biotechnology, 16(2), pp. 132–139. https://doi.org/10.21859/ijb.1454.
Kumar, G., Sindhu, S.S., Kumar, S. and Vanlalruati, V., 2017. In vitro isolation, regeneration and purification of yellow mutant in chrysanthemum (Chrysanthemum morifolium) cv. Lalit through ray floret regeneration. Indian Journal of Agricultural Sciences, 87 (7), pp. 958-963.
Lim, K.B., Kwon, S.J., Lee, S.I., Hwang, Y.J. and Naing, A.H., 2012. Influence of genotype, explant source, and gelling agent on in vitro shoot regeneration of chrysanthemum. Horticulture Environment and Biotechnology, 53(4), pp. 329–335. https://doi.org/10.1007/s13580-012-0063-x.
Liu, Y., He, M., Dong, F., Cai, Y., Gao, W., Zhou, Y., Huang, H. and Dai, S., 2019. The chrysanthemum lavandulifolium clnac9 gene positively regulates saline, alkaline, and drought stress in transgenic chrysanthemum grandiflora. Journal of the American Society for Horticultural Science, 144(4), pp. 280–288. https://doi.org/10.21273/JASHS04697-19.
Lowe, J.M., Davey I’, M.R., Power’, J.B. and Blundy, K.S., 1993. A study of some factors affecting Agrobacterium transformation and plant regeneration of Dendranthema grandiflora Tzvelev (syn. Chrysanthemum morifolium Ramat.). Plant Cell, Tissue and Organ Culture, 33, pp.171–180. https://doi.org/10.1007/BF01983231
Mamgain, J., Mujib, A., Syeed, R., Ejaz, B., Malik, M.Q. and Bansal, Y., 2022. Genome size and gas chromatography-mass spectrometry (GC–MS) analysis of field-grown and in vitro regenerated Pluchea lanceolata plants. Journal of Applied Genetics, 64, 1-21. https://doi.org/10.1007/s13353-022-00727-7.
Naing, A.H., Jeon, S.M., Han, J.S., Lim, S.H., Lim, K.B. and Kim, C.K., 2014. Factors influencing in vitro shoot regeneration from leaf segments of Chrysanthemum. Comptes Rendus - Biologies, 337(6), pp. 383–390. https://doi.org/10.1016/j.crvi.2014.03.005.
Prathyusha, N., Suneetha Professor, S.D., Avd, D. and Suneetha, S.D., 2021. In vitro propagation of chrysanthemum (Chrysanthemum morifolium) cultivars from ray floret explants. The Pharma Innovation Journal, [online] 10(10), pp. 415–417. Available at: http://www.thepharmajournal.com
Qi, Y., Liu, Y., Zhang, Z., Gao, J., Guan, Z., Fang, W., Chen, S., Chen, F. and Jiang, J., 2018. The over-expression of a chrysanthemum gene encoding an RNA polymerase II CTD phosphatase-like 1 enzyme enhances tolerance to heat stress. Horticulture Research, 5(1), pp. 37. https://doi.org/10.1038/s41438-018-0037-y.
Ranadev, P., Kumari, V.R. and Praveen Ranadev, C., 2019. Interaction effect of bio-inoculants on modulating growth and yield of chrysanthemum (Dendranthema grandiflora) cv. yellow gold. Journal of Pharmacognosy and Phytochemistry, [online] 8(5), pp. 622-626. Available at: http://www.phytojournal.com
Simao, M., Fonseca, E., Garcia, R., Mansur, E., and Pacheco, G., 2016. Effects of auxins and different culture systems on the adventitious root development of Passiflora pohlii Mast. and their ability to produce antioxidant compounds. Plant Cell Tissue and Organ Culture, 124, pp. 419–430.
Sjahril, R., Haring, F., Riadi, M., Danial Rahim, M., Sher Khan, R., Amir, A. and R, T.A., 2016. International Journal of Agriculture System (IJAS) Performance of NAA, 2iP, BAP and TDZ on Callus Multiplication, Shoots Initiation and Growth for Efficient Plant Regeneration System in Chrysanthemum (Chrysanthemum morifolium Ramat.). International Journal of Agricultural System, 4(1), pp. 52–61.
Steffens, B. and Rasmussens, A., 2016. The physiology of adventitious roots. Plant Physiology, 170, pp. 604–617.
Su, J., Jiang, J., Zhang, F., Liu, Y., Ding, L., Chen, S. and Chen, F., 2019. Current achievements and future prospects in the genetic breeding of chrysanthemum: a review. Horticulture Research, 6 (109), pp. 2-19. https://doi.org/10.1038/s41438-019-0193-8.
Teixeira Da Silva, J., 2014. Organogenesis from chrysanthemum Dendranthema x grandiflora (Ramat.) Kitamura petals (disc and ray florets) induced by plant growth regulators. Article in Asia-Pacific Journal of Molecular Biology and Biotechnology, 22(1), pp.145–151.
Verma, A. and Prasad, K., 2019. Organogenesis and anatomical study of gamma rays induced mutant of chrysanthemum (Chrysanthemum morifolium Ramat.) from ray florets. Research Journal of Biotechnology, 14(3), pp.44–53.
Verma, M., Vishwavidyalaya, R.D. and Kumar Bansal, Y., 2014. Effect of a Potent Cytokinin Thidiazuron (TDZ) on In vitro Regeneration of Hedychium coronarium J. Koenig-A Valuable Medicinal Plant. International journal of Recent Biotehcnology, 2(1), pp- 38-44. https://doi.org/10.13140/2.1.2252.8648.
Verstraeten, Schotte, S. and Geelen, 2014. Hypocotyl adventitious root organogenesis differs from lateral root development. Frontiers in Plant Science, 5, pp. 495. https://doi.org/10.3389/fpls.2014.00495.
Wu, Q., Yang, H., Sun, Y., Hu, J. and Zou, L., 2021. Organogenesis and high-frequency plant regeneration in Caryopteris terniflora Maxim. using thidiazuron. In Vitro Cellular and Developmental Biology - Plant, 57(1), pp. 39–47. https://doi.org/10.1007/s11627-020-10114-8.
Xu, L., Cheng, F. and Zhong, Y., 2022. Histological and cytological study on meristematic nodule induction and shoot organogenesis in Paeonia ostii ‘Feng Dan’. Plant Cell, Tissue and Organ Culture, 149(3), pp. 609–620. https://doi.org/10.1007/s11240-021-02208-x.
Xu, S., Wu, Z., Zhao, J., Zhang, F., Zhang, X., Chen, F. and Teng, N., 2022. High-efficiency Agrobacterium-mediated transformation of chrysanthemum via vacuum infiltration of internode. Ornamental Plant Research, [online] 2(1), pp.1. https://doi.org/10.48130/OPR-2022-0001.
Zhang, K., Jiang, Y., Zhao, H., Köllner, T., Chen, S., and Chen, F., 2020. Diverse terpenoids and their associated antifungal properties from roots of different cultivars of Chrysanthemum Morifolium Ramat. Molecules, 25(9), pp. 2083. https://doi.org/10.3390/molecules25092083.
Zhao, X.-M., Lian, Y.-J., Jin, Z.-L., Zhang, X.-J., Yan, Y. and Fan, S.-J., 2022. Shoot organogenesis and somatic embryogenesis in leaf tissue of Pulsatilla tongkangensis Y.N. Lee & T.C. Lee. Plant Biotechnology Reports, 16(4), pp.389–400. https://doi.org/10.1007/s11816-021-00727-9.
URN: http://www.revista.ccba.uady.mx/urn:ISSN:1870-0462-tsaes.v26i2.44282
DOI: http://dx.doi.org/10.56369/tsaes.4428
Copyright (c) 2023 Guadalupe López Puc, Gerardo Gaspar Tun Góngora, Julia del Socorro Cano-Sosa, Ana Ramos-Díaz, Alberto Uc-Várguez

This work is licensed under a Creative Commons Attribution 4.0 International License.
